 Last updated: December 17th, 2019 4:56 PM
Last updated: December 17th, 2019 4:56 PM
Delhi Drug License
The Drugs Control Department regulates the manufacture and sales of drugs and Cosmetics. The Government has laid down various drug laws under which the State Drug Controller deals with the licensing of both manufacturing and sale premises of drugs & cosmetics. In this article, we look in detail the documents required, fees and the procedure for online payment to obtain Delhi Drug License.Drugs and Cosmetics Act, 1940
Under the Drugs and Cosmetic Act, 1940, the competent authority is authorized to grant Drug license to carry out drugs/medicines or cosmetics relevant businesses. As per the act, the license covers all kinds of drug or cosmetic businesses such as homoeopathic, allopathic excluding the ayurvedic and unani medicines. The manufacturer or seller of the drugs or cosmetics is responsible if any person using the product is affected due to the negligence of the same and is considered an offence under the act. Moreover, the act covers the penalty to be imposed on the licensee for non-renewal of the license after its expiry.Delhi Drug License for Sale
The drug sale license is issued for both retail as well as wholesale purpose for the distribution of drug in India. The issuance of license for drug subjects to conditions attached with the competent person who deals with drugs and the premises (area of pharmacy shop and storage facility).Pre-requisites for sale license:
An applicant applying for drug sale license under the Drugs & Cosmetics Act, 1940 and Rules should provide the following facilities. For Retail/Whole sale of Drugs requires an independent premise with the minimum area of 10sq.m.and height of 2.75 m To obtain the license for retail as well as wholesale of drugs, a premise of not less than 15 sq.m. with height of 2.75 m is necessary to be provided. In addition, the premises should possess adequate storage facilities like cold storage and deep freezer etc. for storage of general drugs as well as specific drugs.Types of License for Sale:
Sale of drugs requires different types of license for specified drugs. The type of drugs for which the sales license is required and the relevant application form are listed below in the table.| S.N | Category | Sale Type | Application Form | License Form |
| 1. | Drugs other than those specified in Schedule C&C (1)&X | Whole Sale | 19 | 20-B |
| Retail Sale | 19 | 20 | ||
| Restricted (General Store) | 19-A | 20-A | ||
| 2. | Drugs specified in Schedule C&C (1) but excluding those specified in Schedule ‘X’ | Whole Sale | 19 | 21-B |
| Retail Sale | 19 | 21 | ||
| Restricted (General Store) | 19-A | 21-A | ||
| 3. | Drugs specified in Schedule ‘X’ | Whole Sale | 19-C | 20-G |
| Retail Sale | 19-C | 20-F | ||
| 4. | Sale of Drugs from motor vehicles (1) Drugs other than those specified in Schedule C&C (1) (2) Drugs specified in Schedule C&C (1) | Whole Sale | 19-AA | 20-BB |
| Whole Sale | 19-AA | 21-BB R-62- D R | ||
| 5. | Homoeopathic Medicines | Whole Sale | 19-B | 20-D |
| Retail Sale | 19-B | 20-C |
Documents Required Drug Sale License
The necessary documents for drug sale license under Delhi Drug license are mentioned below. Documents required for Fresh Application of Retail/Wholesale license:- System generated application form for relevant drug sale (19/19A/19B/19C as applicable).
- Site plan and key plan of the premise
- Constitution of the firm
- In case of company, memorandum and articles of association and Copy of resolution Passed,.
- In case of partner-ship firm - partnership deed, duly attested by Notary Public
- In case of trust / society - Trust deed.
- Photo ID proof of proprietor / partner / director of the firm
- Affidavit regarding non-conviction of Property/Partner/Director as well as the firm under Drugs & Cosmetics Act, 1940.
- Affidavit regarding compliance of MPD 2021, if the premises are located on DDA residential flat / plot /building
- Online fee deposit receipts.
- System generated affidavit from the registered pharmacist/competent person.
- For Retail Sale Registered Pharmacist:
- Proof of qualification i.e. final degree certificate / provisional certificate with mark sheets
- Registration of Delhi Pharmacy Council
- Appointment Letter and Bio-data
- For Wholesale License Competent person:
- Proof of qualification i.e. final degree certificate / provisional certificate with mark sheets
- Experience Certificate
- Appointment Letter and Bio-data.
- Premises:
- a) If Owned:
- b) If Rented:
- System generated application form for relevant drug sale (19/19A/19B/19C as applicable).
- Affidavit regarding non-conviction of Property/Partner/Director as well as the firm under Drugs & Cosmetics Act, 1940.
- For additional Retail Sale license:
- Proof of qualification i.e. final degree certificate / provisional certificate with mark sheets of Registered Pharmacist.
- Registration of Delhi Pharmacy Council.
- Appointment Letter and Bio-data.
- System Generated Affidavit.
- Online fee deposit receipts.
- System generated application form for relevant drug sale (19/19A/19B/19C as applicable).
- Conversion charge receipt issued by MCD with relevant documents in support of commercial use as per MPD 2021 viz; copy showing the Name of notified commercial / mixed use road/street.
- Online fee deposit receipts.
- Proof of qualification i.e. final degree certificate / provisional certificate with mark sheets of Registered Pharmacist.
- Registration of Delhi Pharmacy Council.
- Appointment Letter and Bio-data.
- System Generated Affidavit
- Proof of qualification i.e. final degree certificate / provisional certificate with mark sheets
- Experience Certificate.
- Appointment Letter and Bio-data
- Affidavit by Competent Person (System Generated)
- Revised documents for premises
- a) If Owned: Registered Sale Deed / Registered GPA or Unregistered Sale Deed / unregistered GPA supported with Electricity Bill or Water Bill or property tax receipt.
- b) If Rented: Rent receipt and either Regd. Rent Agreement or Un registered Rent Agreement along with ownership documents of landlord as stated above
- Revised Site Plan
- For grant of a license in Form 20 or Form 21 or both, the premises are of an area of not less than 10 square meters.
- For grant of a license in form 20-B or form 21-B or both, the premises are of an area of not less than 10 square meters and
- For grant of licenses
- In Form 20 or Form 21 or both, and
- In Form 20-B or Form 21-B or both, the premises should be of an area of not less than 15 square meters.
Delhi Drug License for Manufacturing
An applicant applying for grant of licenses for manufacturing of drugs/cosmetics should provide the premises in an approved industrial area and must comply with the relevant provisions of Drugs and Cosmetics Rules, 1945 as applicable for granting the licenses for manufacture of particular type of drugs / cosmetics.Types of Licenses:
| Type of License |
| For manufacturing of drugs in license on Form 25 |
| For manufacturing of drugs in license on Form 25A |
| For manufacturing of drugs in license on Form 25B |
| For manufacturing of drugs in license on Form 25C |
| For manufacturing of drugs in license on Form 25F |
| For manufacturing of drugs in license on Form 28 |
| For manufacturing of drugs in license on Form 28A |
| For manufacturing of drugs in license on Form 28B |
| For manufacturing of drugs in license on Form 28C |
| For manufacturing of drugs in license on Form 28D |
| For manufacturing of drugs in license on Form 28DA |
| For manufacturing of drugs in license on Form 28E |
| For manufacturing of drugs in license on Form 29 |
| For manufacturing of cosmetics in license on Form 32 |
| For manufacturing of cosmetics in license on Form 32A |
| For approval of Testing Laboratories on Form 37 |
Requisites for Drugs License for manufacturing of drugs
The documents necessary for drug manufacturing license under Delhi Drug License are as follows.- Application in the prescribed form
- Declaration form
- Key plan and site plan in blue print / ammonia print.
- Proof of Possession: A) Rented: rent receipt & rent agreement. (Notary Attested) B) Self-occupied
- Proof of constitution of firm in case of partnership / private limited/ trust.
- Copy of Resolution passed by Board of Directors.
- Proof of Ownership : Copy of registered GPA / sale deed / property tax receipt
- Affidavit of Non Conviction as per format given in Guide Line Booklet.
- List of Equipment And Machineries Provided For Manufacturing.
- List of equipment provided for testing.
- List of technical staff for manufacturing along with their self certified photocopies, certificates of education qualification, experience certificate, biodata on proforma, appointment letter, three photographs.
- List of technical staff for testing along with their self certified photocopies w.r.t. certificates of education qualification, experience certificate, biodata on proforma, appointment letter, three photographs.
- Affidavit from employer and or technical staff for full time working with the firm as per format given in Guide Line Booklet.
- List of Formulations to be permitted along with product information separately for each drug and affidavit about owner / user of the trade name applied for.
- Consent of approved testing laboratory for sophisticated testing.
- All industries seeking licenses for manufacturing of Drugs should obtain a No Objection Certificate from Delhi Pollution Control Committee.
- Drugs Manufacturers Units applying licenses where the drugs involve the use of alcohol and other inflammable material in the processing should acquire a No Objection Certificate from Delhi Fire Services.
Competent Authority
- For Sales License: Licensing Authority of the concerned Zone
- For Manufacturing: Assistant Drugs Controller of the concerned Zone
Time Frame
For Sale License:| Process | Time Period |
| For grant of licenses for sale of drugs or for disposal of application. (if the conditions are satisfied) | 35 days |
| Change addition/ deletion of Registered Pharmacist. | 60 days |
| Change addition/ deletion of Competent Person. | 90 days |
| For grant of sale licenses for Homoeopathic Medicines or for disposal of application. (if the conditions are satisfied) | 90 days |
| For grant of sale licenses for Homoeopathic Medicines or for disposal of application. (if the conditions are satisfied) | 35 days |
| For grant of sale licenses for Schedule X Drugs Medicines or for disposal of application. (if the conditions are satisfied) | 35 days |
For Drug Manufacturing License:
The period for grant of licenses for manufacturing of Drugs / Cosmetics is 75 days from the date of receipt of application, if conditions are satisfied.Application Procedure
- Every application is required to be ‘e-send’ (ESEND is a service to make it easy for you to move files from one account to another account) as it will not be allowed in person to the office.
- For this purpose, the applicant has to obtain the user ID and password by requesting online with the details of the firm.
- All documents must be scanned in 100 dpi & black and white, and then uploaded using the generated user id and password.
- All the original documents are necessary to be showed to the inspector at the time of inspection.
- After the grant or rejection of the application, you will receive an SMS about the same.
- Take a print out of the approval/rejection from ‘Print License’ option for fresh license and renewal and click ‘MISC approvals’ for other approvals within 3 days.
- You will receive licenses/approvals with the valid e-signature and official seal.
E-Payment for License
The applicant can make the payment of fee for the required license through the e-payment portal of the NCT Delhi. Step 1: To make the requisite fee, visit the e-payment website of the Delhi government. Step 2: Select the department as ‘Drugs Control’ and select the required license type for which the payment should be made. Step 3: Enter the required payee details. The amount to be paid for each type of license appears. Step 4: Click submit and proceed with the payment.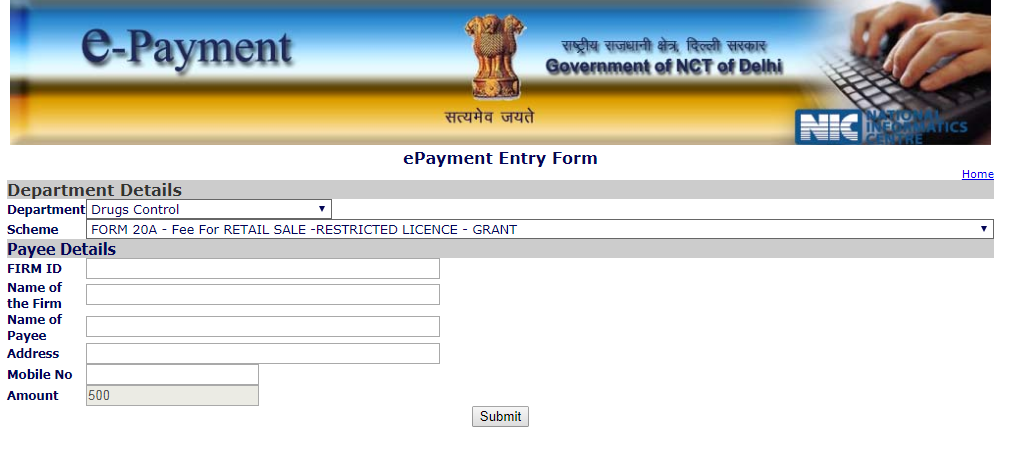 Delhi Drug License - e-Payment
Delhi Drug License - e-Payment
Popular Post

In the digital age, the convenience of accessing important documents online has become a necessity...

The Atalji Janasnehi Kendra Project that has been launched by the Government of Karnataka...

The Indian Divorce Act governs divorce among the Christian couples in India. Divorce...

When an individual has more than a single PAN card, it may lead to that person being heavily penalised, or worse,...

Employees Provident Fund (PF) is social security and savings scheme for employee in India. Employers engaged...


