 Updated on: December 17th, 2019 6:04 PM
Updated on: December 17th, 2019 6:04 PM
Madhya Pradesh Drug License
In India, carrying out any business activity requires an appropriate license. Drug license issued by the Government is a legal permit to conduct businesses that deal with drugs and cosmetics. Drug license is region specific and location specific, i.e. if the business operation occurs in two or more states, then each state requires a different license, and if sold at more than one place, a separate license must be acquired. The drug license comprises drug manufacturing license and drug sale license. In this article, we look at the procedure of obtaining Madhya Pradesh Drug License in detail.Drugs and Cosmetics Act, 1940
Drug license is a permit granted by the competent authority to carry out a business concerning drugs/medicines or cosmetics under the Drugs and Cosmetic Act, 1940. As per the act, the license covers all kinds of drug or cosmetic businesses including ayurvedic, homoeopathic, allopathic or Unani drugs. The manufacturer or seller of the drugs or cosmetics is responsible if any person using the product is affected due to the negligence of the same and is considered an offence under the act.Conditions of License
- The licensee should have at least four years of practical experience in the distribution of drugs.
- Any drug made by the licensee must be under the direct and personal supervision of a registered pharmacist.
- The retail supply of any drug on the prescription of the registered medical practitioner should be under the supervision of a registered pharmacist.
- The retail supply of the drug should be recorded in a register at the time of supply. The following particulars should be made in the register.
- The serial number of the entry
- Date of supply
- Name and address of the prescriber
- Name and address of the patient; if the drug supplied is for veterinary use, name and address of the animal's owner
- Name of the drug and the quantity
- In case of a drug specified in Schedule C or Schedule H, the name of the drug manufacturer, batch number and the date of expiry
- Signature of the registered pharmacist under whose supervision the medicine was supplied
- The licensee should maintain the purchase record of the drugs intended for sale or sold by retail. The record should include the following particulars.
- Date of purchases
- Name and address of the person from whom the drug was purchased and the license number held by him
- Name of the drug, quantity and the batch number
- Name of the manufacture of the drugs
- The drugs specified in Schedule H or Schedule X should be supplied to registered Medical Practitioners, hospitals, dispensaries and nursing homes only on the signed order in writing, which is valid for a period of two years.
- The licensee should not sell or stock any drug after the expiry date.
- The licensee should not store or stock any drugs on his premises that are intended for distribution as a free sample to the medical profession.
- The medicine in a retail shop for the treatment of animals should be labeled as “Not for human use _ for treatment of animals only.”
Madhya Pradesh Drug Sale License
The drug sale license is issued for both retail as well as wholesale purpose for the distribution of drug in India. The issuance of license for drug sale subjects to conditions attached with the competent person who deals with drugs and the premises (area of pharmacy shop and storage facility).Documents Required
For New Drug Sale License
The documents required for obtaining a new drugs sale license are mentioned below. The applicant should duly attest the documents. a) Documents needed for Applicant- Proof of Identity as specified in the online application
- Applicant’s qualification document - Mark Sheet (SSC, HSC & Graduation) as applicable.
- Residential Proof.
- Map of Shop/Firm Premises.
- Shop/Firm related documents (in the case of a firm, partnership deed, in case of a private limited, firm MOA and COI, limited list of directors etc.)
- Proof of ownership/rent agreement with the actual landowner.
- Affidavit of the applicant.
- Purchase bill of the refrigerator.
- Electricity bill of shop/firm premises.
- Two passport size photographs.
- Two passport size Photographs.
- Identity proofs as specified in the online application
- Applicant’s qualification document - Mark Sheet (SSC, HSC & Diploma or Graduation) as applicable.
- Proof of residence
- Appointment Letter of Pharmacist /Competent Person.
- Registration Certificate of Pharmacist in case of retail sale license application
- Pharmacist Registration Certificate and Experience Certificate in case of the wholesale license application
- Copy of drugs sale license along with the Affidavit of the person who issued the Experience Certificate.
- Affidavit of Pharmacist / Competent Person (If Applicant / Pharmacist / Competent person are same, a combined affidavit must be submitted)
- Consent Letter of Pharmacist / Competent Person.
- Registered sale deed / conveyance deed / registered GPA in the name of the owner should be submitted
- In the case of unregistered sale deed / unregistered GPA, it must be supported with water bill / electricity bill / property tax receipt.
- In the case of a village, Khasra Khatauni documents are accepted.
- Registered rent agreement with rent receipt must be furnished. In case of unregistered rent agreement or owner document, No Objection Certificate from the owner of the premises should be produced.
- Site plan and key plan of the premises duly attested by an approved architect or mapmaker of the municipality.
Fees Structure
For New Drugs Sale License
| S.No. | License Type | Fee | Portal Fee |
| 1 | Retail sales drug License | ||
| a) Form 20 | Rs. 1500 | Rs. 150 | |
| b) Form 21 | Rs. 1500 | ||
| c) Form 20-F | Rs. 500 | ||
| 2 | Whole sales drug License | ||
| a) Form 20-B | Rs. 1500 | Rs. 150 | |
| b) Form 21-B | Rs. 1500 | ||
| c) Form 20-G | Rs. 500 | ||
| 3 | Retail and Whole sales drug License | ||
| a) Form 20 | Rs. 1500 | Rs. 150 | |
| b) Form 21 | Rs. 1500 | ||
| c) Form 20-B | Rs. 1500 | ||
| d) Form 21-B | Rs. 1500 | ||
| e) Form 20-F | Rs. 500 | ||
| d) Form 20-G | Rs. 500 | ||
| 4 | Restricted and whole sales drug License | ||
| a) Form 20-B | Rs. 1500 | Rs. 150 | |
| b) Form 21-B | Rs. 1500 | ||
| c) Form 20-A | Rs. 500 | ||
| d) Form 21-A | Rs. 500 | ||
| e) Form 20-G | Rs. 500 | ||
| 5 | Restricted License | ||
| a) Form 20-A | Rs. 500 | Rs. 150 | |
| b) Form 21-A | Rs. 500 | ||
For Drugs Sale License Renewal
| S.No. | License Type | Fee | Portal Fee |
| 1 | Retail sales drug License | ||
| a) Form 20 | Rs. 1500 | Rs. 100 | |
| b) Form 21 | Rs. 1500 | ||
| c) Form 20-F | Rs. 500 | ||
| 2 | Whole sales drug License | ||
| a) Form 20-B | Rs. 1500 | Rs. 100 | |
| b) Form 21-B | Rs. 1500 | ||
| c) Form 20-G | Rs. 500 | ||
| 3 | Retail and Whole sales drug License | ||
| a) Form 20 | Rs. 1500 | Rs. 100 | |
| b) Form 21 | Rs. 1500 | ||
| c) Form 20-B | Rs. 1500 | ||
| d) Form 21-B | Rs. 1500 | ||
| e) Form 20-F | Rs. 500 | ||
| d) Form 20-G | Rs. 500 | ||
| 4 | Restricted and whole sales drug License | ||
| a) Form 20-B | Rs. 1500 | Rs. 100 | |
| b) Form 21-B | Rs. 1500 | ||
| c) Form 20-A | Rs. 500 | ||
| d) Form 21-A | Rs. 500 | ||
| e) Form 20-G | Rs. 500 | ||
| 5 | Restricted License | ||
| a) Form 20-A | Rs. 500 | Rs. 100 | |
| b) Form 21-A | Rs. 500 | ||
Late fee charge for Drugs Sale License Renewal
| S.No. | License Type | Fee |
| 1 | Retail sales drug License | |
| (a) Form 20 | 2% of Fee per month | |
| (b) Form 21 | 2% of Fee per month | |
| 2 | Whole sales drug License | |
| (a) Form 20B | 2% of Fee per month | |
| (b) Form 21B | 2% of Fee per month | |
| 3 | Retail and Whole sales drug License | |
| (a) Form 20 + Form 20B | 2% of Fee per month | |
| (b) Form 21 + Form 21B | 2% of Fee per month | |
| 4 | Restricted and whole sales drug License | |
| (a) Form 20A + Form 21A | 2% of Fee per month | |
| (b) Form 20B + Form 21B | 2% of Fee per month | |
| 5 | Restricted License | |
| (a) Form 20A | 2% of Fee per month | |
| (b) Form 20B | 2% of Fee per month |
Online Application Process for Drug Sale License
The applicant can apply online for Madhya Pradesh Drug Sale License through the official website of the Food and Drugs Administration. Step 1: To apply online, visit the official website. Under the tab ‘Drug Sale license’, click ‘Apply for New License’. Madhya Pradesh Drug License - Drug Sale license
Step 2: The online application form for the New Drug Sale license appears. Enter the required details that includes the following.
Madhya Pradesh Drug License - Drug Sale license
Step 2: The online application form for the New Drug Sale license appears. Enter the required details that includes the following.
- Application Details
- Select the application type as new drug sale license
- Applicant Personal Details
- Applicant Local / permanent address details
- Applicant Qualification Details
- Registered Pharmacist’s Details
 Madhya Pradesh Drug License - Drug Sale license application form
Step 3: Click Submit.
Madhya Pradesh Drug License - Drug Sale license application form
Step 3: Click Submit.
Renewal of Drug Sale License
The application for the renewal of drug sale license can be done by entering the licensee details and the application number. Click Search. The license application can be renewed by updating the changes.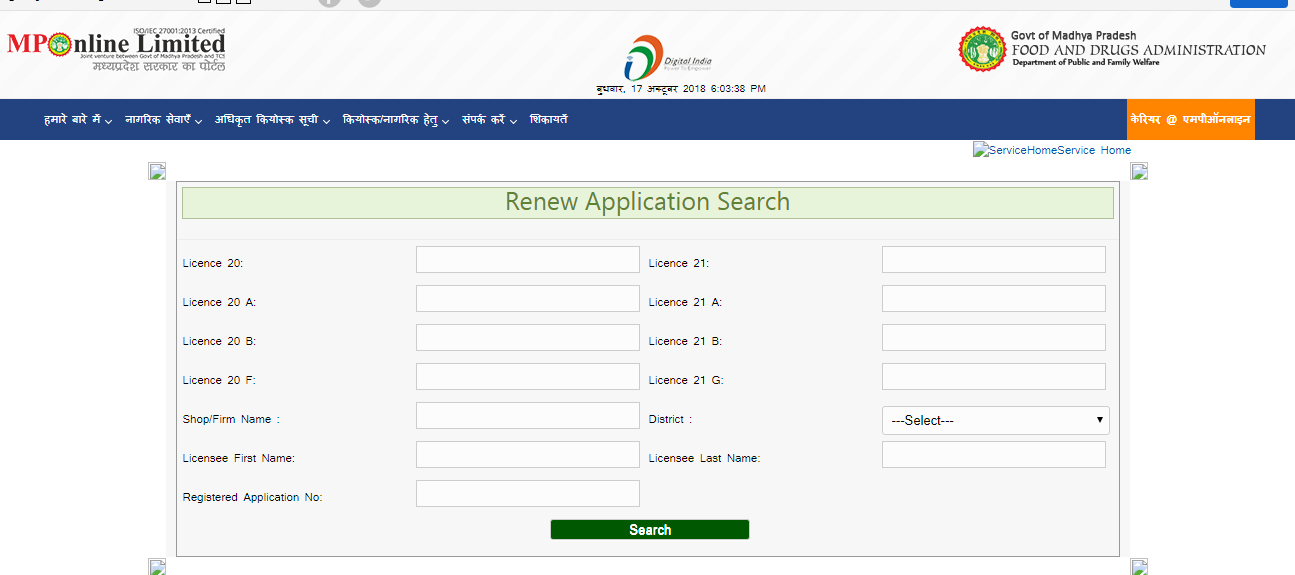 Madhya Pradesh Drug License - Drug Sale license Renew application
Madhya Pradesh Drug License - Drug Sale license Renew application
Popular Post

In the digital age, the convenience of accessing important documents online has become a necessity...

The Atalji Janasnehi Kendra Project that has been launched by the Government of Karnataka...

The Indian Divorce Act governs divorce among the Christian couples in India. Divorce...

When an individual has more than a single PAN card, it may lead to that person being heavily penalised, or worse,...

Employees Provident Fund (PF) is social security and savings scheme for employee in India. Employers engaged...


