 Last updated: December 17th, 2019 4:56 PM
Last updated: December 17th, 2019 4:56 PM
Tripura Drug License
Tripura drug license is a document of permission for carrying out businesses related to drugs, medicines and cosmetics. The government of Tripura issues drug license to regulate the manufacture, storage, distribution and sales of drugs. Tripura drug license is issued under the Drug and Cosmetics Act, 1940 to a person dealing with drugs. As per this act, it is mandatory to obtain a Tripura drug license before the launching a drug business. In this article, we look at the procedure for obtaining Tripura drug license in detail. Know more about How to start a pharmacy businessDrugs and Cosmetics Act
Tripura drug license is a crucial document according to the Drugs and Cosmetics Act, 1940. This act moderates the manufacture, distribution import and sale of drugs and cosmetics, which includes allopathic drugs, homoeopathic medicines, and even the Ayurvedic, Siddha or Unani drugs.Importance of Tripura Drug license
Some of the significance of Tripura drug license is explained in detail below:- The Tripura drug license permits market, manufacture sale, resale, trade of drug and cosmetics.
- Without a Tripura drug license, no person can do pharmacy business or even start to associate with any dealings in Tripura.
- For protecting good health of India through adequately accessing the medicines and drugs, the Drugs and Cosmetics act made it mandatory for obtaining a drug license
- Tripura drug license also ensure that the drug and drugs are not misused or abused by any individuals in Tripura
- Dealing with drugs/medicines a without a drug license is a cognizable offence. Every trader in the pharmacy industry needs to have their drug incense to carry out their day-to-day business work.
- Tripura drug license contains the rule and regulation for the person who is dealing with drugs business
Who needs Tripura Drug License?
Tripura drug license is crucial for dealing with the following type of business. Any proprietorship, partnership firm, limited liability company, company or business firm located In Tripura and is physically distributing the medicine in the state is required to hold a drug license:- Business with any cosmetics intended to be sprinkled, rubbed, poured or introduced into the human body or for beautifying, cleansing, promoting attractiveness, or altering the appearance of a human, must obtain Tripura Drug license.
- It is vital to get a drug license to deal with the drug which is used for internal or external in human beings
- Manufacturing and distribution drugs or cosmetics applied to the human body to repel insects like mosquitoes also need Tripura drug license.
- Business with drugs used for the destruction or insects which cause diseases in human beings must get a drug license.
- Tripura drug license also mandatory for dealing with empty gelatin capsule.
- To manufacture, store and distribute the drugs used for diagnosis, mitigation, treatment or prevention of disease or disorder in humans or animal, need Tripura drug license
Basic Requirement for Obtaining Tripura Drug License
According to the provision of Drug and cosmetics act, to get a Tripura drug license the below-described requirements to be met by the trader.Area Recruitment
The minimum area required for setting up a medical shop or pharmacy is ten square meter. In case of the store business combination with the wholesale and retail of drugs then the minimum area of fifteen square meters is required. License for the sale of drugs can be issued in the commercial premises or other premises independent of residence.Storage Facility
It is crucial to have an air conditioner and refrigerator on the premises because some medicines such as insulin injections, vaccines are needed to be stored in the refrigerator.Technical Staff
Sale of drugs should be carried in the presence of a registered Pharmacist approved by the Tripura Government who is required throughout the working hours in the retail shop. The wholesale of drugs can be made either by a registered pharmacist or any competent authority who graduate and having experience in drugs or in the presence of an of person who has passed SSLC having experience of four years in drugs/medicines business, specially approved by Health and Family Welfare Department.Classification of Tripura Drug License
Based on the requirement of the pharma business, Tripura Drug License can be classified in the following categories:- Sale License
- Manufacturing License
Sale License
Sale license is issued to persons or agencies engaged in the sale of drugs or medicines in Tripura. The sale drug license is further classified into the following two types.- Retail License: It is granted to run and sell drugs or drug products in Tripura.
- Wholesales License: It is provided to a person or agency engaged in wholesale of medicine or drugs
Manufacturing License
Manufacturing license is granted to a person or agencies involved in the manufacturing of drugs, medicines and cosmetics. The government of Tripura also issued Manufacturing loan license.Licensing Authority
The health and family welfare department, Government of Tripura issues manufacturing drug license as per the provisions of drug and cosmetics act. State Drugs Control Directorate issues the license for sale of drugs and cosmetics. The prescribed authority for issuing Tripura drug license is Drugs Controller.Time Frame
Tripura drug license will be issued within 45 days from the date of application.Validity Details
The Tripura drug license granted to carry out business related to the drug is valid for five years from the date of issue. After expiry, the license can be renewed.Documents Required
Documents required for Obtaining Tripura Drug license is as follows:Documents for New Manufacturing Drug License
- Applications in the prescribed form
- Approved plan copy
- Wholesale license copy
- Challan of rupees as prescribed to be paid under the correct head of account
- Draft labels
- Method of analysis
- Flow sheet
- Manufacturing process
- Standard operating procedures
- List of products
- Stability data for each product
- Tripura Sale Tax Clearance Certificate
Documents for New Manufacturing Drug License
- Application Form
- Additional information form
- Partnership deed or MOA (Memorandum of Article of Association) and List of Directors
- Appointment Letter of the Registered Pharmacist
- Consent letter of the registered pharmacist with Details of the Previous Service
- Copy of registration certification issued by Tripura State Pharmacy Council
- Renewal Receipt of Tripura State Pharmacy Council
- Photographs of the Pharmacists
- An Affidavit of the pharmacist on Rs.20 Stamp paper
- Rent Receipt for the Premises and necessary proof for the legal possession of the Premises like Copy of the registered Sales Deed
- Rough Sketch of the Premises
- Ration Card or PAN Card or identity card or Aadhar card of all Partners or Directors and Pharmacist
Tripura Drug License (Retail and Wholesale) Application Procedure
The procedure to obtain Tripura sale drug license Tripura is explained in step-by-step procedure here:Access XLN INDIA
Step 1: The applicant has to access the XLN INDIA web-portal. From the home page select Tripura from the drop-down menu and click on sales login button.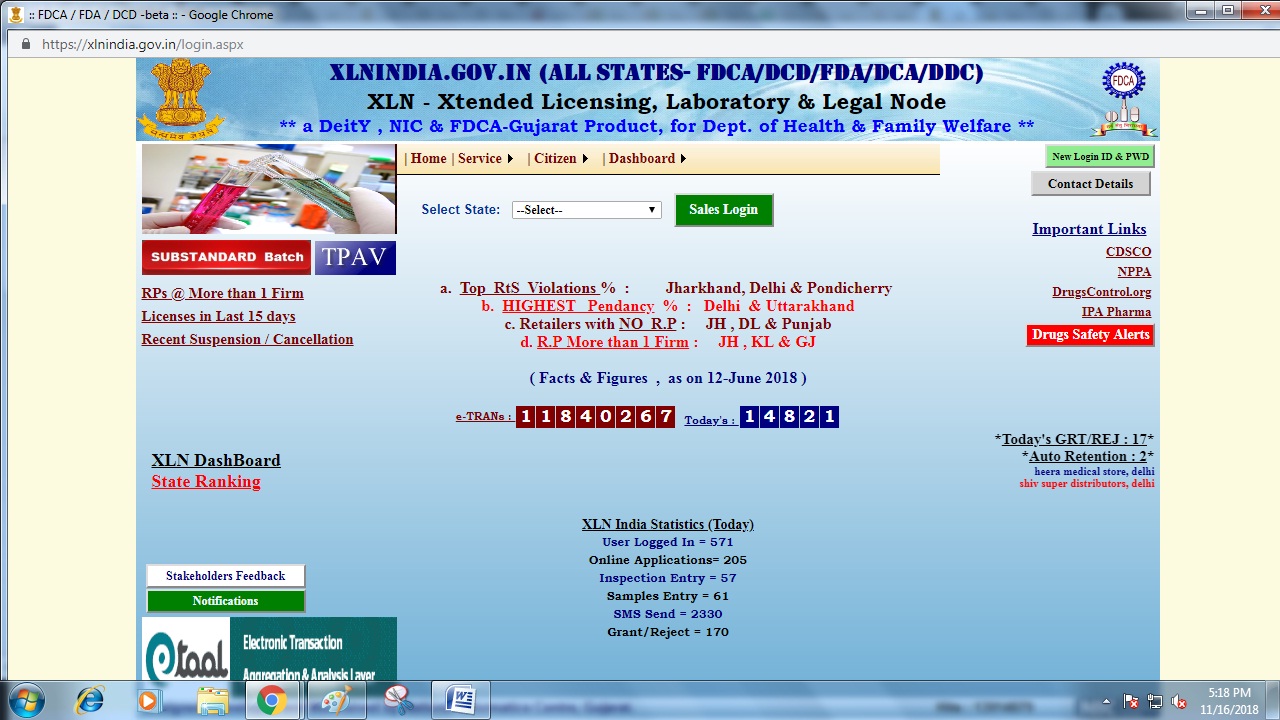 Image 1 Tripura Drug License
Image 1 Tripura Drug License
Registration
Step 2: The link will redirect to the registration page. In this page, you have to apply for the User ID and Password; you will get the user login credential on the registered mobile number.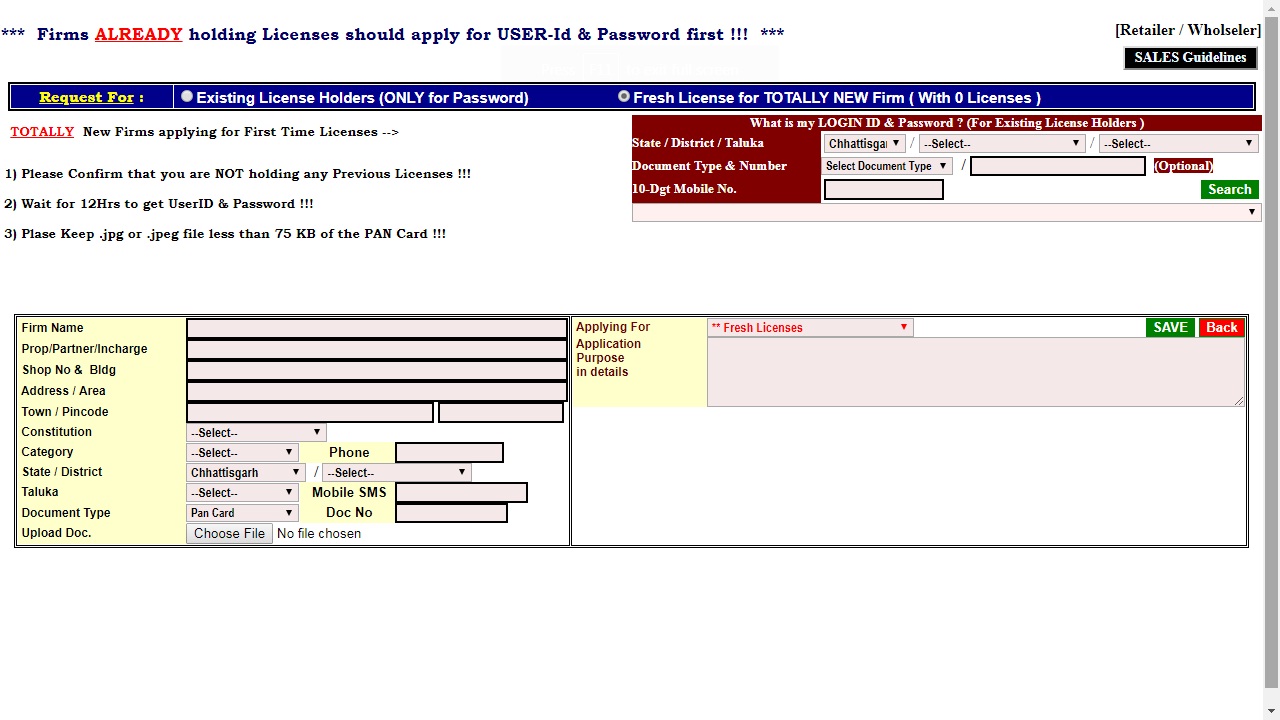 Image 2 Tripura Drug License
Step 3: After obtaining user login credential, login to the web-portal by entering the username and password. Click on the submit option.
Image 2 Tripura Drug License
Step 3: After obtaining user login credential, login to the web-portal by entering the username and password. Click on the submit option.
Login to Portal
Step 4: Once you log in the portal, you can change the password for security and privacy purpose. The option for modifying the password is available on the right top corner of home page.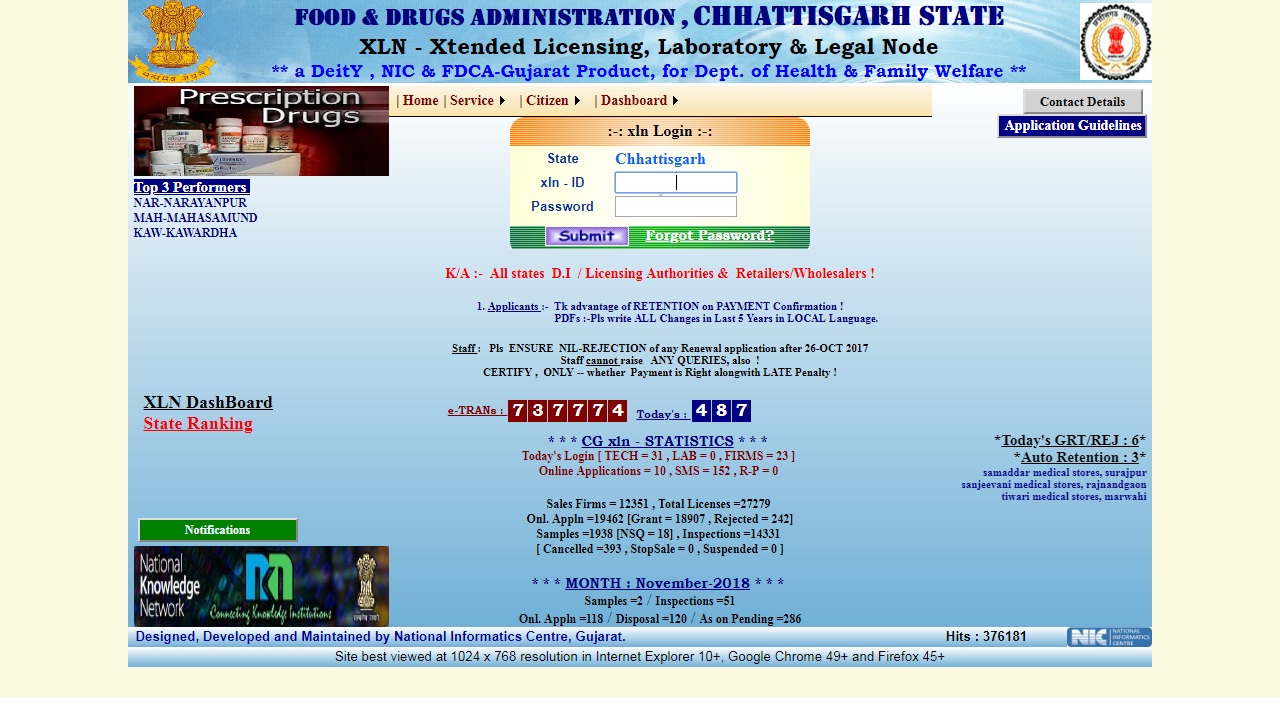 Image 3 Tripura Drug License
Step 5: After providing details, click on update my details and correct it, if needs. Once the application is corrected, click on the update option to proceed further.
Image 3 Tripura Drug License
Step 5: After providing details, click on update my details and correct it, if needs. Once the application is corrected, click on the update option to proceed further.
Provide Details
Step 6: Select the prepare or edit online application option for providing details. Step 7: After selecting the new inward option, click on the OK button. Step 8: Select the Storage type from the drop-down list. Note: You have to select at least one option from this list. Step 9: You have to select the document type from the drop-down list and select the document as an Aadhaar card and provide the Aadhaar number in the space provided. Step 10: You have to select the inward type option from the drop-down list and select free-add/Fresh license option. Click on the RP option in Retail and CP in Wholesale button. Step 11: By clicking on the Add option, you can provide all details for Tripura drug license.Upload Documents
Step 12: Upload all supporting documents. The certificate should consist the registration certificate of a pharmacist from Tripura pharmacy tribunal and current renewal. After uploading the documents, click on the save option. Step 13: You need to provide all other details by clicking on the CON option and then on the add option. Step 14: Take the print out of the self-declaration of CP or RP and proprietor. Get it signed by the registered pharmacist and proprietor or authorised signatory. Step 15: By following the method described above, you can upload all the other required documents when needed. Step 16: After uploading the documents, select the back button. In this new page, click on by firm and print application form. Note: Keep the application safe for uploading after making payment. You need to upload all otherPayment of Fee through E-payment Gateway
Step 17: The applicant can make the payment through the e-payment gateway option available in the portal. Once the payment is successful, you can take the print out of the e-challan.Upload Challan
Step 18: After receiving the challan, login to the portal for uploading the e-Challan. After login to the portal, Click on by firm option. Select payment entries and click on New. Step 19: Select payment type as FRAS and furnish the required details. Upload the scanned copy of deposited challan using ‘Choose file’ button and click on save option. Step 20: Select the upload option for uploading application and all other documents, after uploading click on the Close button.Additional Information
Step 21: By selecting the additional information or undertaking option, provide all details and click on the sign button. Select the additional form button and then select the back button.Forward Application to licensing authority
Step 22: You need to Certify and e-Send Application to Department, i.e. the licensing authority. Licensing authority will forward the application to concerned drug inspector within five days for verification.Site Verification by Drug Inspector
Step 23: The drug inspector will verify the document and premises, and drug inspector will submit his report to the licensing authority within 15 working. Step 24: After viewing the report, the concerned licensing authority will grant or reject the drug license within a few days.Status of Application
Applicant should log in to the portal frequently to check the status of the application in the dashboard. The status will also be available in the inbox to check if any query raised by the department, you can reply accordingly.Print License
By login to the portal, you can print the Tripura drug license. The license should be printed within three days from the date of issue.Tripura Drug License (Manufacturing) Application Procedure
The application procedure for obtaining the Tripura Drug License (Manufacturing) is described in detail here: The government of Tripura is prescribed different Forms for Issuance of Manufacturing License According to the Nature of Drugs. Step 1: You need to submit the application form along with the documents to the District office. Step 2: The drug controller will conduct a field inspection. After verification, drug controller will forward the report to the commissioner. Note: If the application is for more than two categories of products, an inspection conducted jointly by drug controller and district officer. Step 3: The concerned authority reviews the report and submits recommendations to the district officer. The concerned authority will issue the license. The applicant can get the Tripura Drug license from the district office.Popular Post

In the digital age, the convenience of accessing important documents online has become a necessity...

The Atalji Janasnehi Kendra Project that has been launched by the Government of Karnataka...

The Indian Divorce Act governs divorce among the Christian couples in India. Divorce...

When an individual has more than a single PAN card, it may lead to that person being heavily penalised, or worse,...

Employees Provident Fund (PF) is social security and savings scheme for employee in India. Employers engaged...


