 Updated on: November 15th, 2024 3:05 PM
Updated on: November 15th, 2024 3:05 PM
Uttar Pradesh Drug License
Drug license is a legal permit issued by the Government for businesses that deal with drugs and cosmetics. The Food Safety and Drug Administration Department under the Government of Uttar Pradesh grants drug license to prevent the manufacture and sale of sub-standard, fake medicines and to implement the provisions of the Drugs and Cosmetics Act, 1940. In this article, we look at the procedure and various other aspects to obtain Uttar Pradesh Drug License for sales and manufacture in detail.License Conditions
- A registered pharmacist should directly and personally supervise any drug made by the licensee.
- The licensee should have minimum four years of practical experience in the distribution of drugs.
- The retail supply of any drug prescribed by the registered medical practitioner should be under the inspection of a registered pharmacist.
- The retail supply of the drug should be recorded in a register at the time of supply.
- The drugs mentioned in Schedule H or Schedule X are valid for a period of two years and should be supplied to registered Medical Practitioners, hospitals, dispensaries and nursing homes only on the signed order in writing.
- The licensee should maintain the purchase record of the drugs intended for sale or sold by retail.
- The licensee should not sell or stock any drug after the expiry date.
- The licensee should not store or stock any drugs on his premises that are intended for distribution as a free sample to the medical profession.
- The medicine in a retail shop for the treatment of animals should be labelled as “Not for human use _ for treatment of animals only.”
Uttar Pradesh Drug License for Sales
The drug sale license is applicable for both retail as well as the wholesale purpose for the distribution of the drug in India. The license for drug sale is issued based on the conditions subjected to the competent person dealing with drugs and the premises (area of pharmacy shop and storage facility).Types of Drugs Sales License
| Type of Sales License | For grant of license on Form No. | Application to be submitted on Form no. |
| For Wholesale license | Form 20B Form 21B | Form 19 |
| For Retail License | Form 20 Form 21 | Form 19 |
| For Restricted License | Form 20A Form 21A | Form 19A |
| For Drugs specified in Schedule-X(Wholesale) | Form 20G | Form 19C |
| For Drugs specified in Schedule-X(Retail) | Form 20F | Form 19C |
Requirements for Drug Sales License in Uttar Pradesh
The following documents are the pre-requisites for the application of Drug Sales License under Uttar Pradesh Drug License.- Affidavit attested by Public Notary:
- Of Proprietor, if the applicant firm is proprietorship firm.
- Of all the partners, in case the firm is a partnership firm.
- Of the person duly authorized by the Board of Directors of the Private Limited of Limited Company
- Copy of partnership deed if partnership firm
- Address proof of the authorized proprietor / applicant (copy of voter I.D., driving license)
- Affidavit of the liable person for day-to-day working and for any violation of laws pertaining to Drugs.
- Documents related to Pharmacist:
- Affidavit duly attested by Public Notary.
- Copies of educational qualification certificates (Attested)
- Attested copy of registration certificate and its renewal issued by U.P. Pharmacy Council.
- Two photographs per applied license.
- Address proof.
- Appointment letter and joining letter
- Documents related to qualified person (for Wholesale license):
- Attested copies of educational qualification certificates
- Experience certificate on the affidavit
- Two photographs per applied license
- Appointment letter and Joining letter if applicable
- Three copies of the plan of proposed premises.
- Documentary proof of rental or ownership basis of the proposed premises
- Photocopy of the rental agreement in case of rented premises.
- Attested copy of ownership proof of rented premises from the landlord.
- Two photographs of Proprietor/Partners/Authorized person.
- Attested copy of purchase invoice of a refrigerator
Fees
While applying offline, the fees should be paid in the Government Treasury or Government Branch of State Bank of India of the district where the license is required. The original of the treasury challan must be submitted with the application.| Type of Sales License | Fees for Grant of License |
| For Wholesale license | Rs. 1500+1500= Rs. 3000.00 |
| For Retail License | Rs. 1500+1500= Rs. 3000.00 |
| For Restricted License | Rs. 500+500= Rs. 1000.00 |
| For Drugs specified in Schedule-X(Wholesale) | Rs. 500 |
| For Drugs specified in Schedule-X(Retail) | Rs. 500 |
Renewal of Drug Sales License
Renewal of Sale license should be made on the application form same as the form submitted during the grant of the new license along with the necessary fee. The Fee for the renewal of the license is same as the grant of license. The late fee for the renewal of the license is as follows that is applicable up to six months.| Late fee for Renewal Per month |
| Rs. 500+500= Rs. 1000.00 |
| Rs. 500+500= Rs. 1000.00 |
| Rs. 250+250= Rs 500..00 |
| Rs. 250.00 |
| Rs. 250.00 |
Documents Required for Renewal
- Copy of last renewal
- Affidavit of Pharmacist and current rent agreement
- Address proof of the authorised proprietor/applicant
- Affidavit of the liable person for day-to-day working and for any violation of drug laws
Uttar Pradesh Drug License for Manufacture
Types of Manufacturing License:
| Type of Manufacturing License | Application Form to be submitted |
| For drugs other than those mentioned in Schedules C , C (1) and X | Form 24 |
| For Homeopathic Medicines | Form 24C |
| For drugs mentioned in Schedule X and not specified in Schedules C & C(1) | Form 24F |
| For drugs mentioned in Schedules C and C (1) excluding those specified in Schedule X | Form 27 |
| For loan license for drugs mentioned in Schedules C and C (1) excluding those specified in Schedule X | Form 27A |
| For drugs mentioned in Schedules C, C(1) and X | Form 27B |
| For the manufacture of drugs for the purposes of examination, test or analysis | Form 30 |
| For approval for carrying out tests on drugs/cosmetics or raw materials used in the manufacture on behalf of licensees for manufacture for sale of drugs/cosmetics | Form 36 |
Documents Required
The following documents are the pre-requisites for the application of Drug Manufacture License under Uttar Pradesh Drug License.- Affidavit of the following attested by Public Notary:-
- Proprietor of the applicant firm, in case the firm is proprietorship firm.
- All the partners, in case the firm is a partnership firm.
- The person duly authorised by the Board of Directors of the Private Limited or Limited Company.
- In case the applicant Company is a Private Limited or Limited Company, a copy of minutes of the Board of Directors
- If Partnership firm, list of names and addresses of all partners. List of Board of Directors of Private Limited or Limited Company with full names and addresses of all directors.
- Attested copy of registration certificate from District Industries Centre if the applicant firm is a Small Scale Unit.
- Partnership deed copy if partnership firm.
- Address proof of the authorised applicant/proprietor ( Voter I.D, Driving license)
- Documents for Manufacturing Chemist Employed (not applicable if the license is required on Form 37 for a testing laboratory):
- Affidavit duly attested by Public Notary
- Attested copies of educational certificates, experience certificates.
- Attested copy of the letter of approval by State Drugs Controller.
- Copy of consent letter, appointment letter or joining letter.
- Medical certificate from a registered medical practitioner along with the eye fitness certificate.
- Four photographs per applied license
- Address proof
- Documents related to Analytical Chemist Employed:
- Affidavit duly attested by Public Notary
- Attested copies of educational certificates, experience certificates
- Attested copy of the letter of approval by State Drugs Controller
- Copy of consent letter, appointment letter or joining the letter
- Medical certificate from a registered medical practitioner along with the eye fitness certificate.
- Four photographs per applied license
- Address proof
- Attested copy of the consent of Approved testing laboratory that tests those raw materials and finished products which require sophisticated instruments for analysis
- Three copies of the Plan of Premises showing all the sections and dimensions.
- Documentary proof of ownership or rental basis of the proposed premises. Copy of rental agreement in case of rented premises. Attested copy of ownership proof of rented premises from the property owner
- Copy of No Objection Certificate from Pollution Control Board.
- In case of renewal of license attested copy Air and Water Consent from the Pollution Control Board (not applicable if the manufacturing unit is exempted for the same).
- Copy of test report of water from a Government Approved Laboratory regarding its portability and freedom from pathogenic microorganisms
Processing Time
The license will be issued within 15 days from the date of submission of application online.Application Procedure
The Food Safety and Drug Administration Department of Uttar Pradesh provides its citizen with the facility for applying online for Uttar Pradesh Drug License. Step 1: To apply online visit the official website of Food Safety and Drug Administration, Uttar Pradesh. Click ‘Online Drug License Registration and Licensing System’ that redirects to the online application portal. [caption id="attachment_63554" align="aligncenter" width="689"]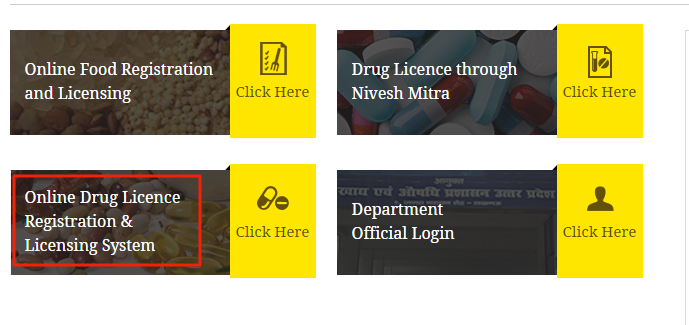 Uttar Pradesh Drug License - Homepage
Step 2: Click ‘Apply online for license’ if registered.
Uttar Pradesh Drug License - Homepage
Step 2: Click ‘Apply online for license’ if registered.
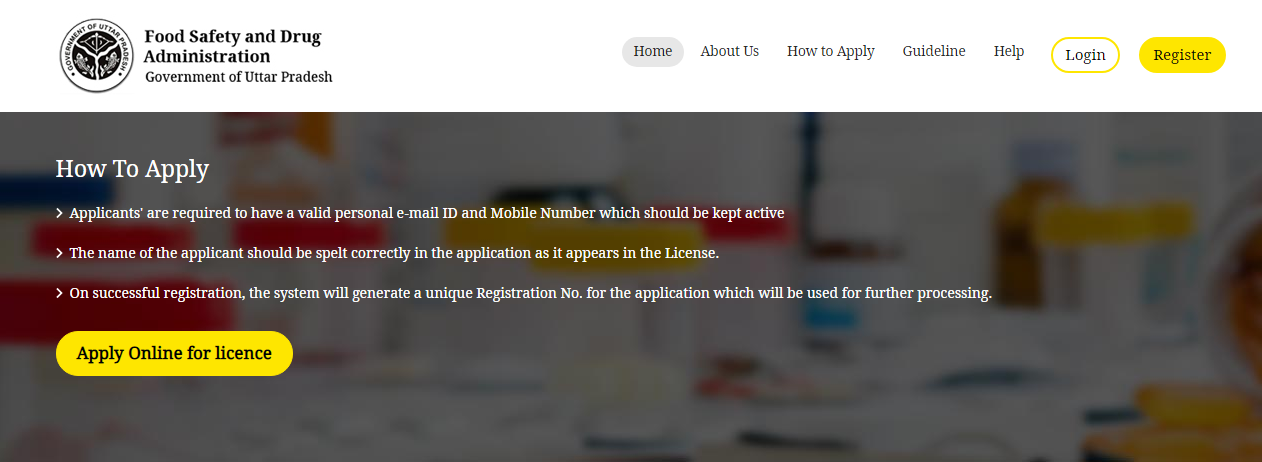 Uttar Pradesh Drug License - Apply online
If not a registered user, click ‘Register’ on the top of the page. One can register as a firm or a technical person.
[caption id="attachment_63556" align="aligncenter" width="391"]
Uttar Pradesh Drug License - Apply online
If not a registered user, click ‘Register’ on the top of the page. One can register as a firm or a technical person.
[caption id="attachment_63556" align="aligncenter" width="391"]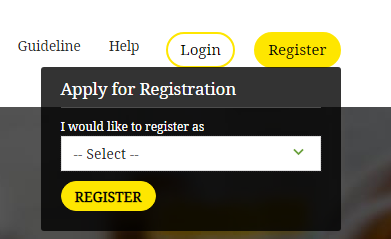 Uttar Pradesh Drug License - Registration
Step 3: Select the registration type; sales unit or manufacturing unit.
[caption id="attachment_63557" align="aligncenter" width="350"]
Uttar Pradesh Drug License - Registration
Step 3: Select the registration type; sales unit or manufacturing unit.
[caption id="attachment_63557" align="aligncenter" width="350"] Uttar Pradesh Drug License - Registration type
Applicants' should have a valid personal e-mail ID and mobile number, which should be kept active for registration.
Step 4: The registration will be validated by entering the Aadhar number for which an OTP will be generated to the registered mobile.
Step 5: Enter the OTP and click Validate.
Step 6: Select the appropriate form of License. Based on License Form, select application Form to be applied.
Step 7: Enter the required details, make the payment and click submit.
Step 8: On successful registration, a unique registration number will be generated which is required for further processing.
Uttar Pradesh Drug License - Registration type
Applicants' should have a valid personal e-mail ID and mobile number, which should be kept active for registration.
Step 4: The registration will be validated by entering the Aadhar number for which an OTP will be generated to the registered mobile.
Step 5: Enter the OTP and click Validate.
Step 6: Select the appropriate form of License. Based on License Form, select application Form to be applied.
Step 7: Enter the required details, make the payment and click submit.
Step 8: On successful registration, a unique registration number will be generated which is required for further processing.
Popular Post

In the digital age, the convenience of accessing important documents online has become a necessity...

The Atalji Janasnehi Kendra Project that has been launched by the Government of Karnataka...

The Indian Divorce Act governs divorce among the Christian couples in India. Divorce...

When an individual has more than a single PAN card, it may lead to that person being heavily penalised, or worse,...

Employees Provident Fund (PF) is social security and savings scheme for employee in India. Employers engaged...


